PUBLICATIONS
First synthetic diamonds brought to LFG
Aurélien Delaunay, Head of the Diamonds Department, French Gemmology Laboratory Emmanuel Fritsch, Professor at the University of Nantes
Synthetic diamonds have been the subject of numerous studies since they were first created in the 1950s. Synthetic techniques have improved and gemstone synthetic crystals have gradually come on the market (Shigley et al., 1986; Shigley et al., 1997; Shigley et al., 2004; Martineau et al., 2004; Wang et al., 2012; etc.). Some synthetic diamonds had already been identified occasionally at other laboratories (see for example Chadwick and Breeding, 2008). For the purposes of scientific analysis, the French Gemmology Laboratory (LFG) gained possession of some synthetic diamonds created by high pressure high temperature (HPHT) and by chemical deposition in the steam phase (CVD: Chemical Vapor Deposition). Their analysis will make it possible for the LFG to increase its database to detect synthetic diamonds. Thanks to this research and familiarisation work, the LFG recently easily detected synthetic diamonds in mixed yellow-brown diamond batches (Delaunay and Fritsch, 2014).
In November 2014, two stones were deposited with the LFG to identify their nature. It was a yellow stone and an almost colourless stone in a marked shade of grey brown. The first (Figure 1a) is a square with cut panels, at degrees, measuring 5.28 x 5.22 x 3.32 mm and weighing 0.15 g (0.72 ct.). The second (Figure 1b) is a round brilliant cut stone measuring 4.74 – 4.85 x 2.68 mm and weighing 0.08 g (0.38 ct.).
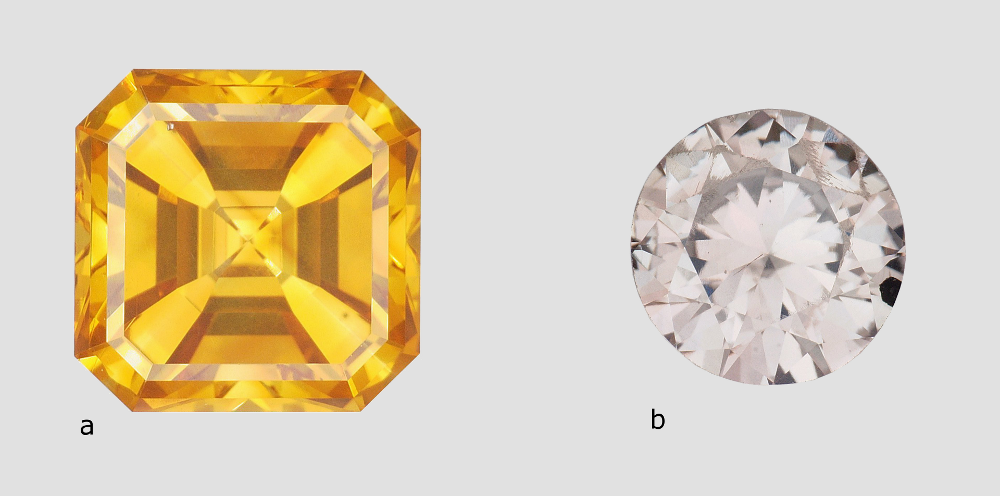
Figure 1: Stones placed with the LFG for analysis weighing 0.72 ct. (a) and 0.38 ct. (b)
The stones were photographed with a Hirox KH-3000 digital microscope for continuous optical magnification up to 320 times and numeric up to 1280 times.
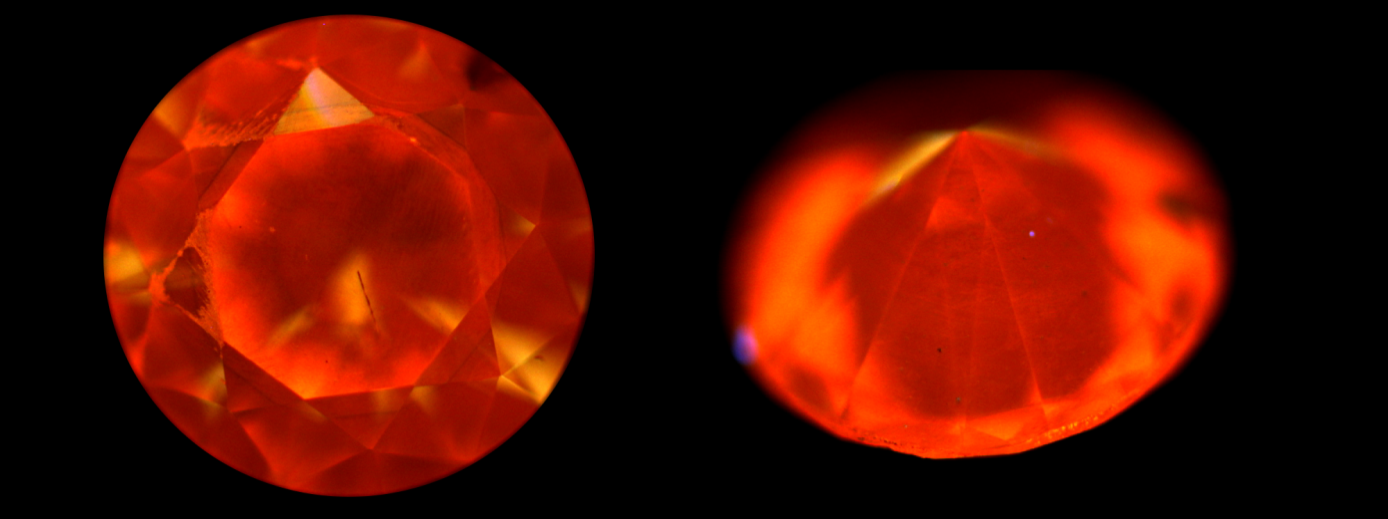
The largest stone contains a multitude of dispersed particles in no apparent order (Figure 2). A cubic metal inclusion can be seen under the stone table (Figure 3). Rifts oriented according to the faces of the octahedron crop up at the breech. Colourless zones set in “cross-formation” can easily be noted in immersion (Figure 4). These properties have often been described for HPHT synthetic diamonds. The colourless areas are areas of dodecahedral growth, not found in natural diamonds. The second stone is remarkable for its mediocre polish, with numerous occurrences of paste on the table and crown. It also contains a graphite inclusion under the crown (visible in figure 1b). In immersion, when viewed between crossed polarizers, the interference figures are particular, made of “cells” and rectilinear areas (Figure 5a). In profile, the parallel sheaves or “brush” appearance is perfectly visible, whatever the direction of observation. This appearance is very similar to that described by Martineau in 2004 on CVD synthetic diamonds (Figure 5b). Such structures are very uncommon in natural diamonds. Thus, from as early as binocular observation, the two stones are strongly suspected of being synthetic diamonds, resulting from two different methods.
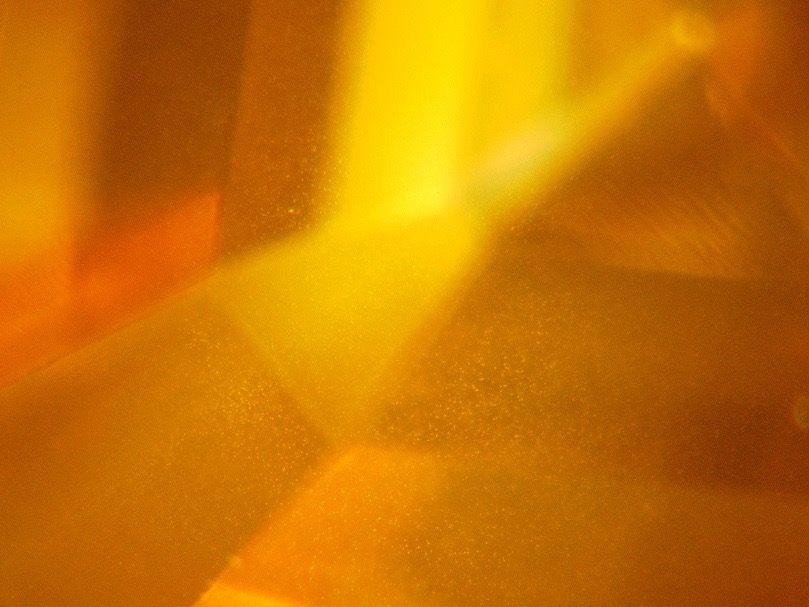
Figure 2: Scattered white dots in the first stone (magnification x160)
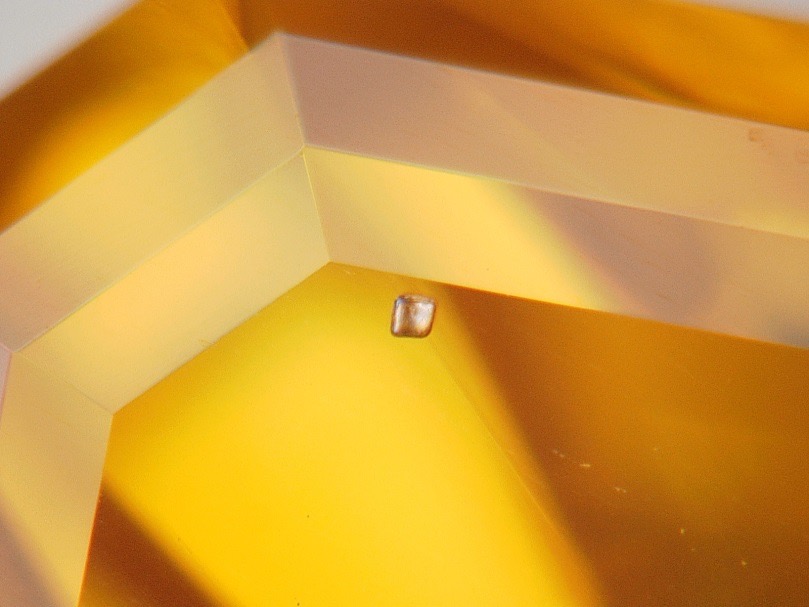
Figure 3: Cubic metal inclusion in the first stone (magnification x160)

Figure 4: Colourless zones distributed in cross formation in the yellow stone (immersion in alcohol, magnification x80)
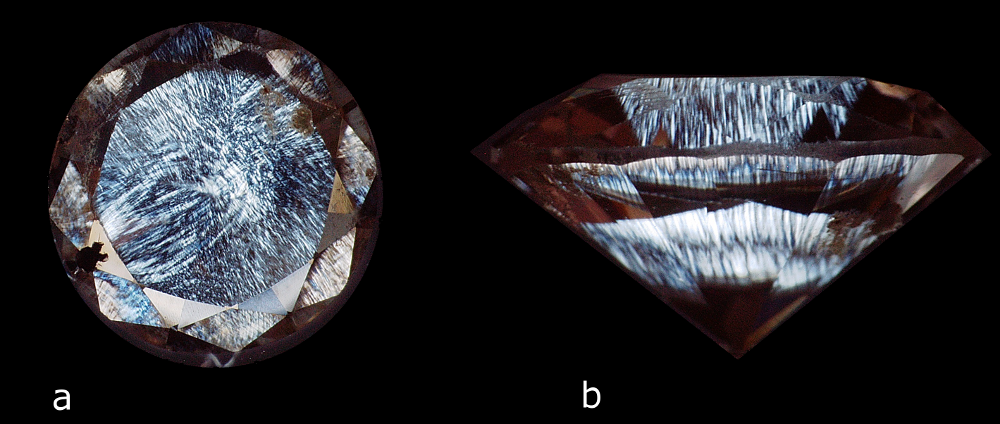
Figure 5: Alveolar and fibrous or “brush” appearance of the second stone observed in immersion in alcohol between crossed polarizers (magnification: a – x40 and b – x60)
Figure 6: Luminescence reactions of the two stones analysed under UVL (365 nm) and UVC (254 nm). The first stone has a zoned green yellow cross-shaped reaction with a slightly more intense reaction to UVC. The second stone is inert in UCL and slightly pink orange in UVC.
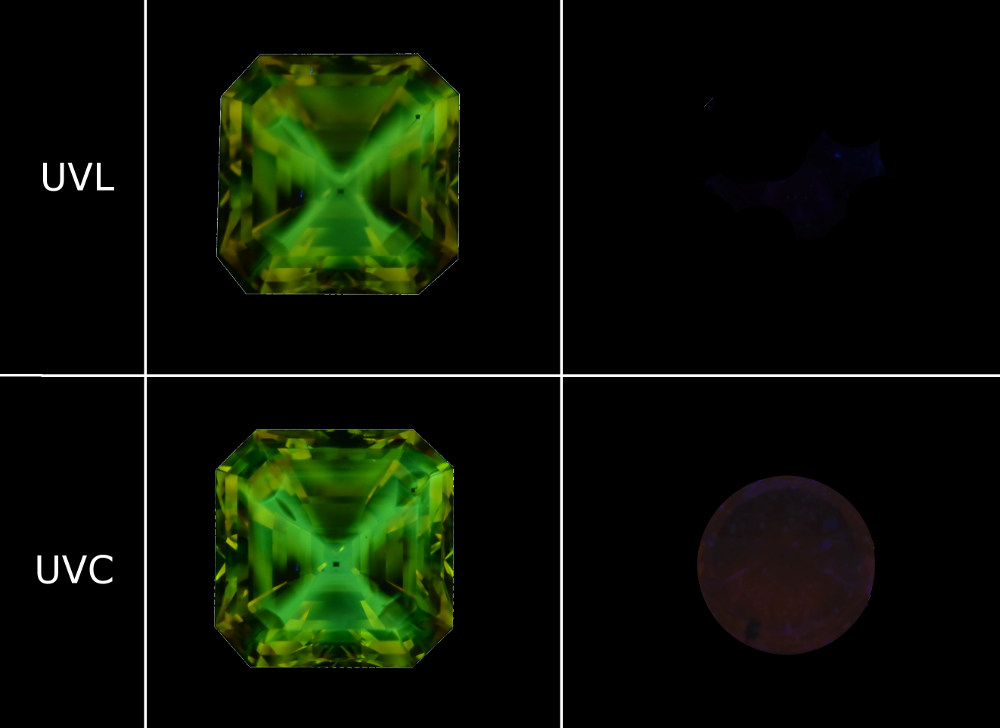
Ultraviolet (UV) luminescence is an important criterion for identifying synthetic diamonds. We used a 6 Watt UVProducts UVGL-58 lamp. The yellow diamond reacts strongly with a zoned yellow-green luminescence (in cross formation) when exposed to ultraviolet long radiation (UVL 365 nm), which is slightly more intense with short ultraviolet radiation (UVC 254 nm). The second diamond is inert when exposed to UVL and slightly pink orange when exposed to UVC (Figure 6). Fluorescence levels that are higher in response to UVC exposure than to UVL exposure offer the gemmologist with an excellent indication that a diamond might be synthetic, particularly in the event of a coloured diamond. This behavior is extremely rare in natural diamonds. Nonetheless, the intensity of the luminescence when exposed to short UV depends greatly on the power of the lamp, the distance between the lamp and the object, and the observation environment: total darkness is recommended to observe low luminescences such as those of the second stone.
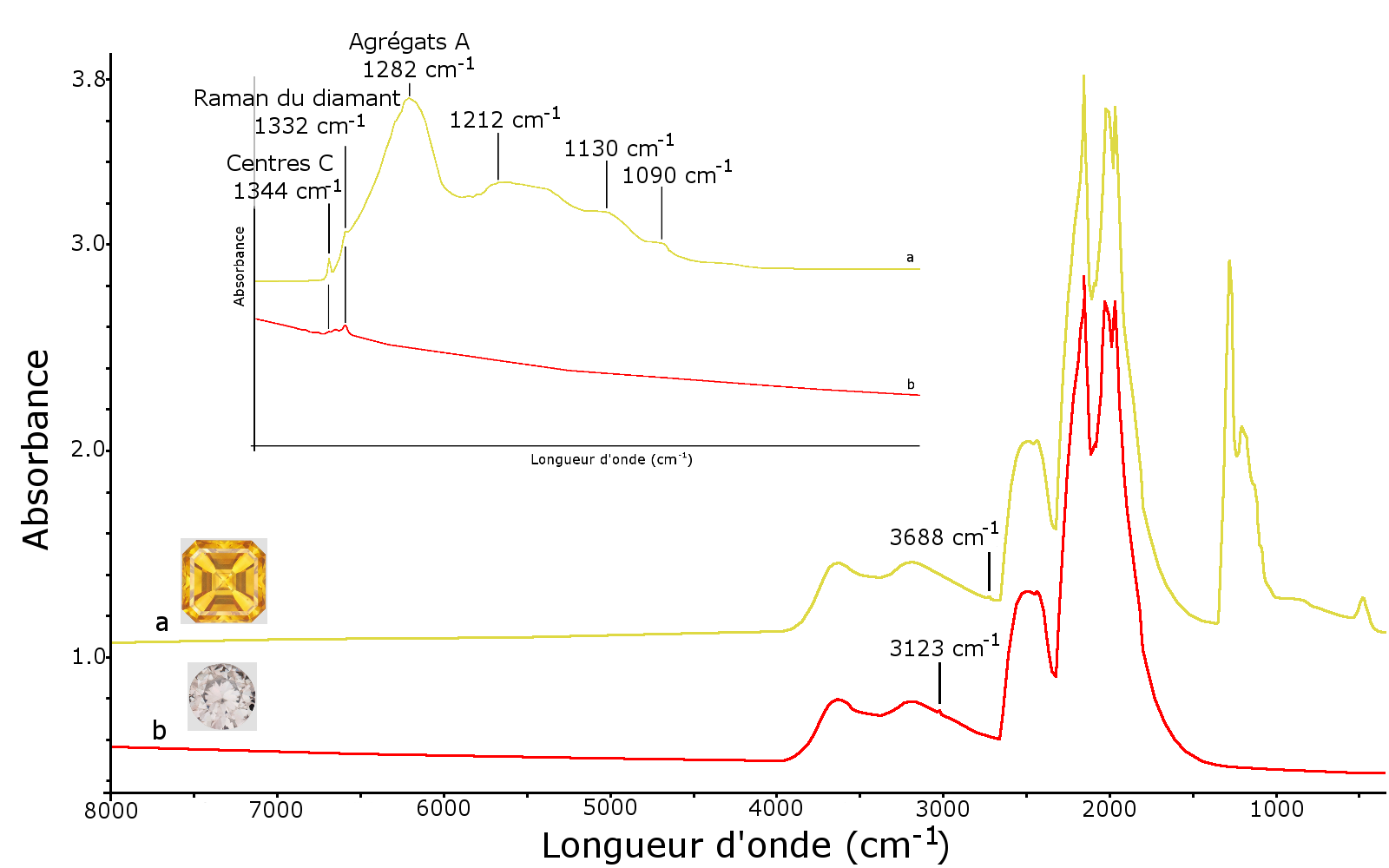
Figure 7: Infrared spectra of the two stones analysed; a – IaA diamond + Ib type showing the bands due to aggregates A at 1282 and 1212 cm-1 as well as centres C at 1090, 1130, 1344 and 2688 cm-1; b – type IIa spectrum with a very low band at 1344 cm-1 due to centre C and at 3123 cm-1 due to complex [N-V-H]0
To characterise the materials, we used Nicolet Magna 560 IR Spectrometer Infrared Fourier Transform. The infrared spectra were obtained from 8,000 to 400 cm-1 at a resolution of 2 cm-1. The first stone is a diamond containing nitrogen in aggregate form (aggregate A or pair of nitrogen atoms at 1282, 1212, 482 cm-1) and in isolated form (centre C at 1344, 1130, 1090, 1045 cm-1). The harmonic at 2688 cm-1 of the band at 1344 cm-1 due to centres C and the Raman band of diamond at 1332 cm-1 are visible in the infrared spectrum (Figure 7). The presence of A aggregates indicates high-pressure high-temperature treatment after growth (Shigley et al., 1993). This is common enough to adjust the often overly-dark colour of synthetic yellow diamonds.
The second stone is a type IIa diamond, i.e. without nitrogen detectable using infrared spectrometry. However, by expanding the scale of the infrared spectrum, a thin band at 1344 cm-1 due to the presence of a few isolated nitrogen atoms, as well as a band at 3123 cm-1 due to the complex [N-V-H]0 (Martineau et al., 2004) can be seen. The absorption at 3123 cm-1 is characteristic of CVD synthetic diamonds
Figure 8: UV-Visible-PIR spectra of the two stones analysed. a – yellow diamond spectrum marked by a band at 377 with a shoulder showing maximum absorption of approximately 464 nm and smaller bands at 529, 555, 690, 715 and 735 nm. The latter having been attributed to the presence of nickel. b – the spectrum of the second diamond with a broad band at 270 nm indicating the presence of isolated nitrogen atoms, another broad band at 688 nm and a narrower band at 737 nm due to the presence of the Si-V centre.
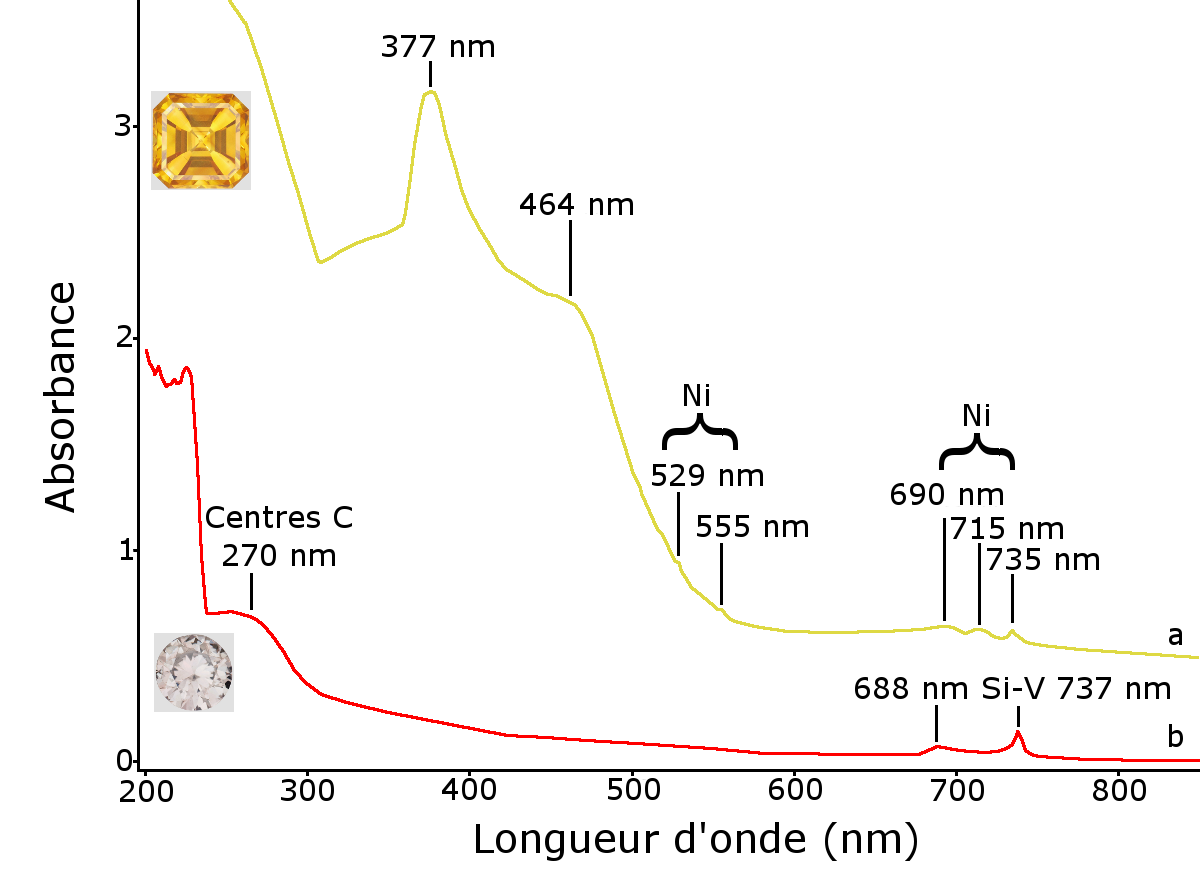
The causes of the colour were analysed using a Jasco V670 infrared UV-Visible-Close spectrometer. The infrared spectra were obtained from 200 to 1000 at a resolution of 1 nm. The yellow diamond’s UV-Visible-PIR spectrum is characterised by an overall increase in the absorption of the near infrared up to the UV, causing the yellow colour. A fairly broad absorption band of approximately 377 nm (approximately 30 nm wide) with a shoulder showing maximum absorption of approximately 464 nm can also be seen. Far less intense and far thinner bands should also be noted at 529, 555, 690, 715 and 735 nm (Figure 8). All of these bands have already been listed in Russian-made HPHT synthetic diamonds with HPHT treatment and have been attributed to the presence of nickel (see p. 241 of Shigley et al., 1993), probably trapped by nitrogen. This type of spectrum, in particular the bands at 377 and 464 nm, has never been documented in natural diamonds. The second diamond’s UV-Visible-PIR spectrum is characterised by absorption at 737 nm due to the Si-V centres as well as one at 688 nm, and a wide band centred at 270 nm due to Centers C (see for example Zaitsev, 2001, pp. 343-344). These bands are often found in CVD synthetic diamonds that are not “ultra pure” (Martineau et al., 2004). The absorption background resulting in a brown-grey colour is linked in the CVD synthetic diamonds to the presence of traces of nitrogen, and therefore entirely consistent with the infrared spectrum.
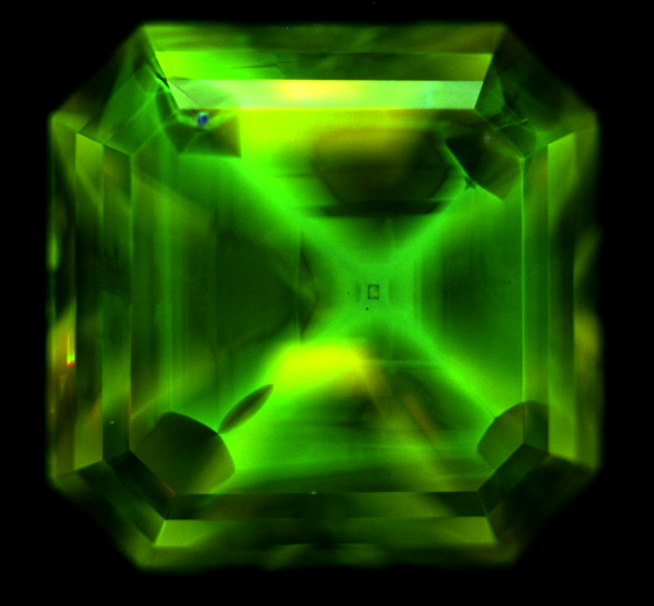
Figure 9: DiamondView image showing cubo-octahedral growth morphology, referred to as the “cross-formation” characteristic of HPHT synthetic diamonds
DiamondView, a very short wave length luminescence imaging device (225 nm), makes it possible to observe diamond growth morphologies. The DiamondView image of the first diamond shows a cubo-octahedral “cross-formation” growth typical of HPHT synthetic diamonds (see for example Figure 9, Shigley et al., 1997). The DiamondView image of the second diamond shows an orange luminescence caused by the presence of N-V0 centres. A striation parallel to the table similar to that of the glass garnet doublets can be seen by looking at the diamond table from above. In profile view, parallel striations can also be noted (Figure 10). These striations are a criterion for recognising CVD synthetic diamonds (Martineau et al., 2004).
Figure 10: DiamondView images of CVD synthetic diamond, orange luminescence is due primarily to N-V centers, the successive layered structure is visible on both images.
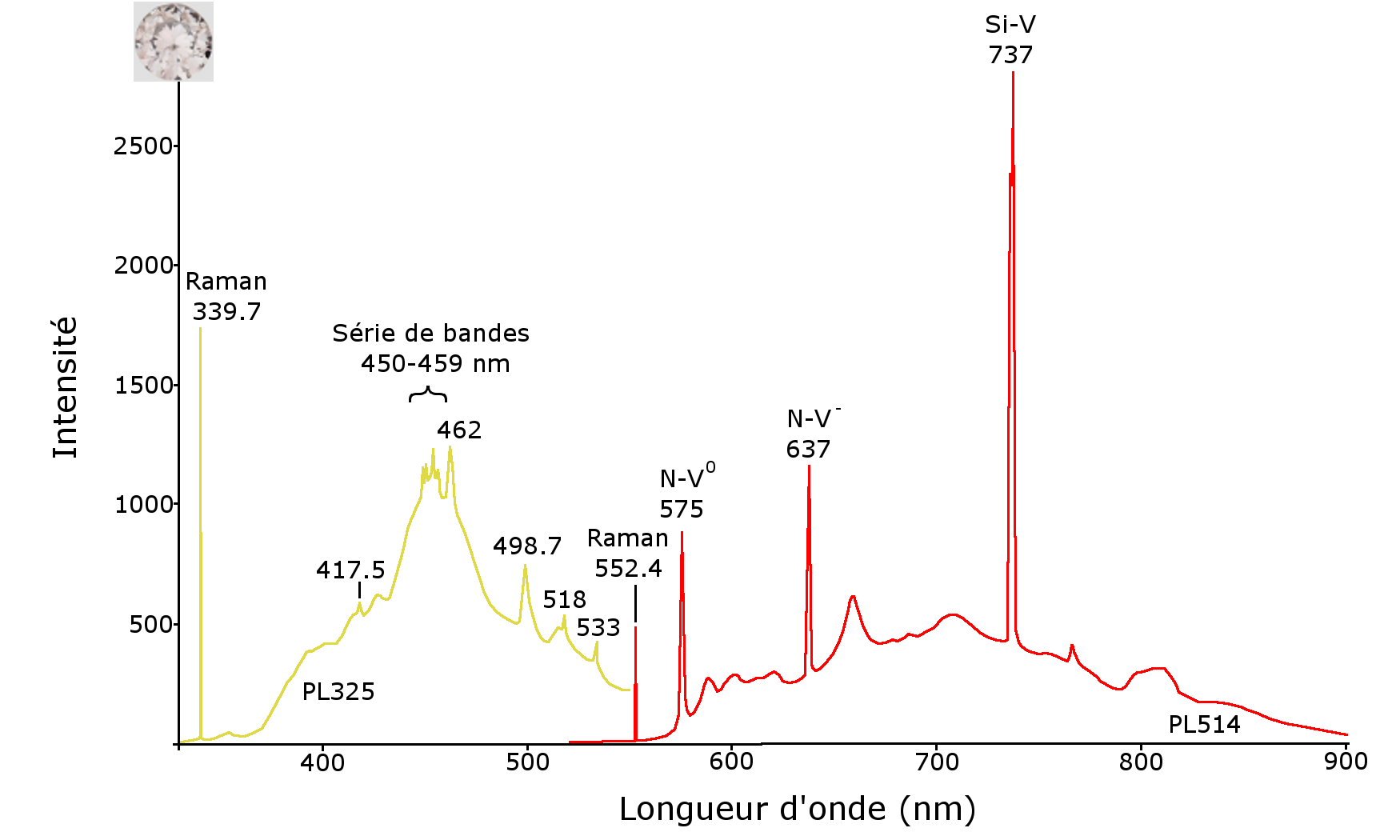
Photoluminescence spectra were generated at liquid nitrogen temperature to confirm the results already ascertained, and to document these crystals more fully. For this purpose, a Raman Renishaw InVia spectrometer equipped with 514 nm and 325 nm lasers was used. HPHT synthetic diamond photoluminescence spectra show multiple peaks, stemming from the presence of traces of nickel trapped by the nitrogen. These are for example found at 478, 489, 497, 523 (a group of bands attributed to centre S2), 727, 747, 808, 869, 883 and 885 nm (Figure 11). The photoluminescence spectra of the CVD synthetic diamond, meanwhile, are strongly marked by the presence of the Si-V centre with a 737 nm doublet, as well as by the presence of the N-V0 and N-V- centres at 575 and 637 nm, respectively. The presence of the Si-V centre is very frequent in CVD synthetic diamonds, while it is very rare in natural diamonds (Breeding and Wang, 2008). A series of peaks, visible between 450 and 462 nm, had also been reported by Martineau and his colleagues in 2004 (Figure 12). This series of bands was observed in CVD synthetic diamonds created on a siliceous substrate from oxygen-containing gases. These bands were attributed to the presence of centres due to oxygen (Zaitsev, 2001). Thus, the laser photoluminescence spectra at liquid nitrogen temperature confirm that the stones submitted are indeed synthetic diamonds, the yellow obtained by HPHT method, and the near-colourless by the CVD technique.
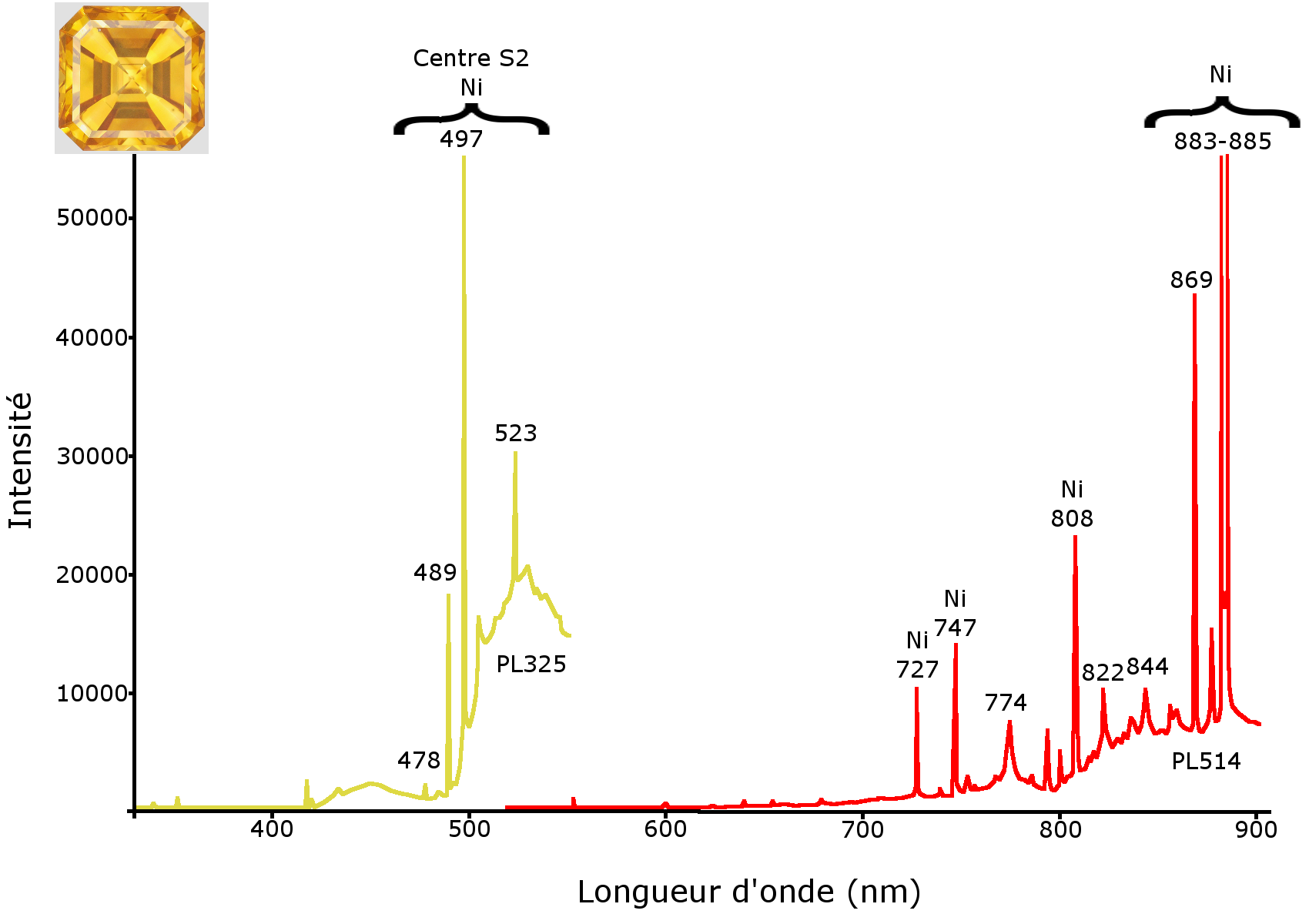
Figure 11: photoluminescence spectra under excitation at 325 nm (PL325, left) and 514 nm (PL514, right) of the yellow synthetic diamond HPHT. The two spectra are strongly marked by the presence of flaws due to nickel, in particular the S2 centre (478, 489, 497 and 523 nm) visible on PL325 and with the bands at 727, 747, 808, 869, 883 and 885 nm on PL514.
This is the first time that isolated HPHT and CVD synthetic diamonds larger than “melee” diamonds are submitted to the French Gemmology Laboratory for analysis. Through classic gemmology, the HPHT yellow synthetic diamond can be easily recognised, for example based on the presence of colourless sectors and metal inclusions. The identification process is more difficult with the CVD synthetic diamond. The short UV transparency points to classification amongst the probably type IIa diamonds, those with a risk of being synthetic or HPHT-treated. With a powerful UVC lamp, the low orange luminescence when exposed to UVC can also be detected, giving reason to suspect CVD synthesis. Observation of the “brush structure” in immersion between cross polarisers is sufficient to confirm this suspicion.
Figure 12: photoluminescence spectra under excitation at 325 nm (PL325 on the left) and 514 nm (PL514 on the right) of the CVD synthetic diamond. The Si-V 737 nm center is often characteristic of CVD synthetic diamonds. The series of bands between 450 and 462 nm has already been documented in CVD synthetic diamonds, and appears to be due to the presence of oxygen in the gases used for synthesis.
References
- Breeding C.M., Wang W., 2008, Occurrence of the Si-V defect center in natural colorless gem diamonds, Diamond and Related Materials, vol. 17, pp. 1335-1344
- Chadwick K.M., Breeding C.M., 2008, First CVD synthetic diamond submitted for dossier grading, Gems & Gemology, vol. 44, n°1, pp. 67-69
- Delaunay A., Fritsch E., 2014, Two synthetic diamonds identified in a parcel of 7000 melee-size yellow-brown diamonds, Journal of Gemmology, Vol. 34, n°1, pp.
- Martineau P.M., Lawson S.C., Taylor A.J., Quinn S.J., Evans D.J.F., Crowder M.J., 2004, Identification of synthetic diamond grown using chemical vapor deposition (CVD), Gems & Gemology, vol. 40, n°1, pp. 2-25
- Shigley J.E., Fritsch E., Stockton C.M., Koivula J.I., Fryer C.W., Kane R.E., 1986, The gemological properties of the Sumitomo gem-quality synthetic yellow diamonds, Gems & Gemology, vol. 22, n°4, pp. 192-208
- Shigley J.E., Fritsch E., Koivula J.I., Sobolev N.V., Malinovski I.Y., Pal’yanov Y.N., 1993, The gemological properties of Russian gem-quality synthetic yellow diamonds, Gems & Gemology, vol. 29, n°4, pp.228-248
- Shigley J.E., Moses T.M., Reinitz I., Elen S., McClure S.F., Fritsch E., 1997, Gemological properties of near-colorless synthetic diamonds, Gems & Gemology, vol. 33, n°1, pp. 42-53
- Shigley J.E., Breeding C.M., Shen A.H.-T., 2004, An updated chart on the characteristics of HPHT-grown synthetic diamonds, Gems & Gemology, vol. 40, n°4, pp. 303-313
- Wang W., D’Haenens-Johansson U.F.S., Johnson P., Moe K.S.M., Emerson E., Newton M.E., Moses T.M., 2012, CVD synthetic diamonds from Gemesis Corp., Gems & Gemology, vol. 48, n°2, pp. 80-97
- Zaitsev A.M., 2001, Optical properties of diamond, Springer-Verlag, Berlin, 502 p.



